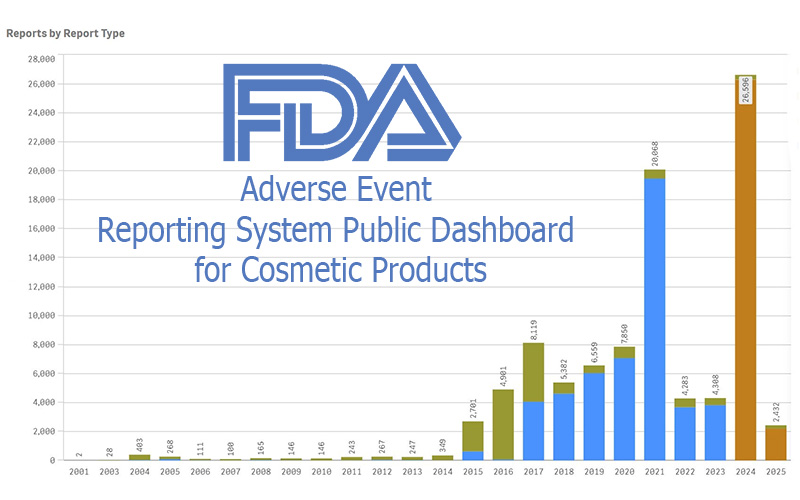
On September 12, 2025, FDA launched the FDA Adverse Event Reporting System (FAERS) Public Dashboard for Cosmetic Products. (Yes, that’s a mouthful!) It’s a searchable, downloadable, daily-updated window into adverse-event reports tied to cosmetics sold in the U.S.
This is new. Until now, the information on adverse events from cosmetics was buried in with the drugs and biologics, was updated quarterly, and was HARD to find.
Why a Public Dashboard?
First, it’s part of the agency’s “radical transparency” push. The FDA also just recently made a similar dashboard of real-time drug and biologic reports publicly available.
Second, it follows on MoCRA. One aspect of MoCRA was the requirement to report any severe adverse reactions from a cosmetic product to the FDA. That went into effect in the end of 2023. It was new–prior to MoCRA, adverse event reports were voluntary, not required. Now, with the new FAERS public dashboard, we have a way to see what those reports have been.
Key Definitions
First, there are some key words to know:
Serious Adverse Event:
(A) An adverse event that results in either:
- a life-threatening experience;
- inpatient hospitalization;
- a persistent or significant disability or incapacity;
- a congenital anomaly or birth defect;
- an infection;
- or significant disfigurement (including serious and persistent rashes, second- or third-degree burns, significant hair loss, or persistent or significant alteration of appearance), other than as intended under conditions of use that are customary or usual; or
(B) A medical or surgical intervention, based on reasonable medical judgment, to prevent an outcome described in (A) above.
Responsible Person:
The manufacturer, packer, or distributor of a cosmetic product whose name appears on the label of such cosmetic product.
What’s in the Dashboard
The dashboard contains more than just the required serious adverse event reports. There is, and has been for years, a way for cosmetic companies, health care professionals, and consumers to voluntarily report any adverse events (serious or not) — and all those reports are included as well.
What’s really important to keep in mind is that these are unverified reports.
In fact, before you can even view the dashboard, there is a very long disclaimer that you have to accept. It states that just because a report exists, it doesn’t mean that the report is valid or that there is any proof that the product actually caused the adverse event. Nonetheless, the reports are included for public view.
Honestly, the dashboard is extremely difficult to navigate and search. Mostly it’s statistics of various categories (date, sex, gender, seriousness). It’s complicated to drill down to actual products and companies. They are there, but hard to find and very hard to actually browse through.
Why it’s Important – Pros & Cons
The PROs
- Transparency. Consumers, advocates, and media can now see what kinds of adverse events are being reported for individual cosmetic lines. That kind of visibility can build trust — especially if there aren’t any reports for your products!
- Research / Trends. Going forward, the data in the dashboard will be used by the FDA and others to find commonalities between products that have been reported. If you want to take the time, you could drill down to find any products similar to yours that have been reported, and see if there is anything that affects you. Either way, it may provide an “advanced warning system” for potential issues.
Given that over 90% of the serious reports in the database appear to be concerning talc-containing powders, it certainly shows how it could have been (or maybe was) a warning system for the dangers of talc.
The CONs
- Raw data. The FDA explicitly warns: the presence of a report is not proof that the cosmetic caused the event. Reports are unverified, may contain duplicates or incomplete information, and therefore can’t be reliably used to establish incidence rates. But in the court of public opinion, many won’t read the disclaimers. That raises reputational risk.
- “Trial by dashboard.” A lawyer might search for reports that can be used as the basis for initiating potential (money-making) individual or class action lawsuits.
- Unjustified consumer fear or overreaction. A handful of rash claims tied to a particular type of product, then picked up by the media, could alarm consumers. Similar products could be caught up in the frenzy. (And this is often where legislators get the idea for more laws.)
The Reality
Assuming that your products are well-made, have a good preservative system as needed, and don’t contain any unsafe ingredients, the likelihood of a consumer filing an adverse reaction report is slim to none. Of the 26,596 reports received in 2024, only 326 (about 1.2%) were from consumers. The rest were required serious adverse event reports filed by the responsible person.
What You Should Do Now
What you should do now is what you should be doing anyway.
- Make safe products (required by law and good common sense)
- Keep good records of your manufacturing process (GMP)
- Track your batches (GMP)
- Maintain safety substantiation for your cosmetic products and their ingredients (required by MoCRA)
- Have a system in place, with good record-keeping, to handle any customer complaints quickly so they don’t become adverse events reported to the FDA .
Special Note for Soapmakers
If you only make “soap that is not a cosmetic” (labeled and marketed solely as soap for cleansing), then MoCRA’s cosmetic serious adverse event reporting rules and this dashboard do not directly apply.
However, you are still required to report any serious adverse events to the Consumer Product Safety Commission.
Final thoughts
This dashboard is a big shift: more sunlight on cosmetic safety, fewer barriers to the data.
For small makers, that sunlight can grow trust—or magnify misunderstandings—depending on how you prepare.
My advice: treat the dashboard as your radar. Build light-weight systems that fit your size to keep all your basics in place, so you never have to deal with a report about your product in the dashboard.
Transparency is here to stay. Let’s use it to show that even the smallest brands can lead when it comes to safety.

Leave a Reply
You must be logged in to post a comment. Login/Register