-
![FDA Postmarket Regulation of Cosmetic Products [Video]](https://www.mariegale.com/wp-content/uploads/2026/02/FDA-Grand-Rounds-2026-02-05.webp)
FDA Postmarket Regulation of Cosmetic Products [Video]
This month the FDA Grand Rounds focused on MoCRA: “Postmarket Regulation of Cosmetic Products: The Who, What, Where, When, Why and How.”
-

Cause Marketing for the Holiday Season
The holiday shopping season is a perfect time to showcase your products AND your values. That makes cause marketing an appealing option for both customers and marketers.
-

Labeling: Gift Baskets & Sets
Gift baskets are great product opportunities, but they have label requirements, just like any other product.
-
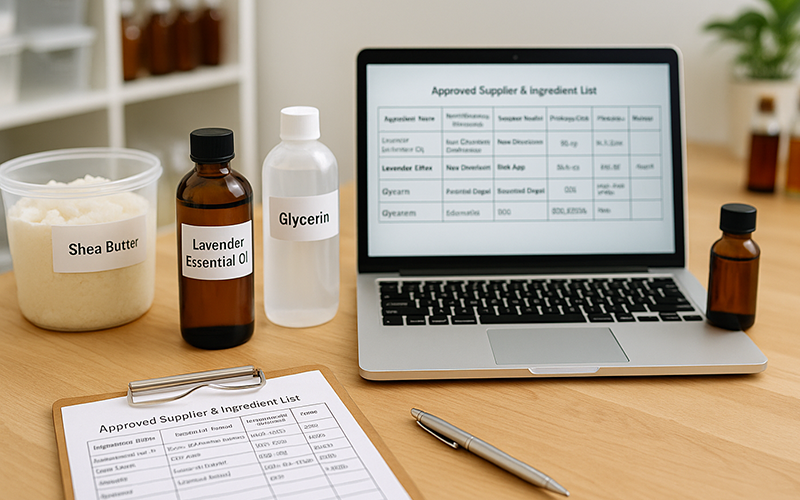
GMP: Tracking Ingredients
Tracking ingredients through every step — from specifications and suppliers to receiving and use — is a core part of GMP. Here’s how to make it simple, sustainable, and fit into your workflow.
-

“Bug-Off” Soaps and Lotions Are Pesticides
“Bug-off” soaps and lotions may look like skincare, but if they claim to repel insects, they’re regulated as pesticides—even when made from safe, natural ingredients.
-

Handcrafted Sunscreen? Nope.
Sunscreens are OTC drugs and must meet ingredient, testing, and manufacturing requirements. Non-sunscreens for use when tanning require a warning label.
-

Good Manufacturing Practices: What’s the Point?
You might wonder, What’s the point of going to all the trouble of following good manufacturing practices? After all, your products are great, right?
-

Alcohol in Cosmetics
Ethyl, denatured, or isopropyl — what’s really in your product? Not all alcohols are the same, and they have different regulations and labeling requirements.
-

Safety Substantiation: What it Means (and How To Do It)
Before MoCRA, a cosmetic product was supposed to be safe, but there was no requirement for proof of its safety. Now proof is required.
-
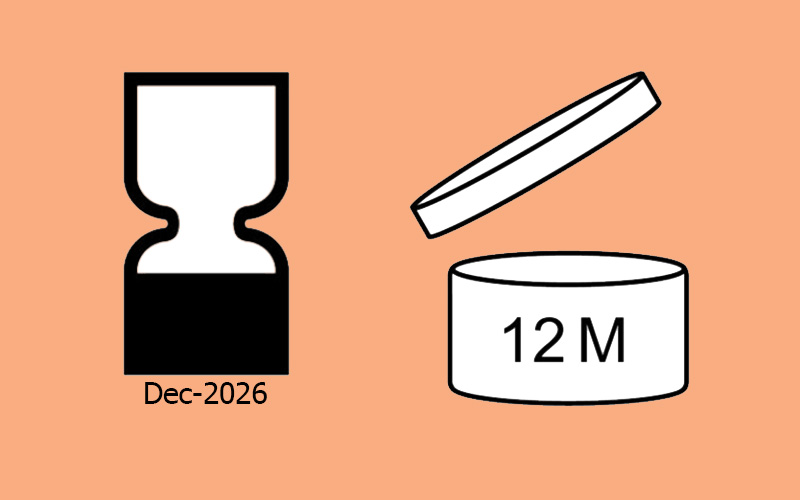
Using the Open Jar and Hourglass Symbols
The open jar and hourglass symbols appear on many cosmetic labels—but in the U.S., they’re often misunderstood or misused. Here’s what they actually mean.
-

Glass vs. Plastic: Which Wins?
Choosing between glass and plastic isn’t just about looks. Explore the real trade-offs in sustainability, cost, safety, and perception — and find what fits your products best.
-

Incidental Ingredients: Declare or Not?
Incidental ingredients are trace materials with no function in a cosmetic. Here’s when you can omit them—and why transparency still matters.
