-

Scented Products: Asset or Risk?
Fragrance can attract or repel customers. Learn how to choose balanced scents, respect scent-free policies, and offer fragrance-free options for wider customer appeal.
-
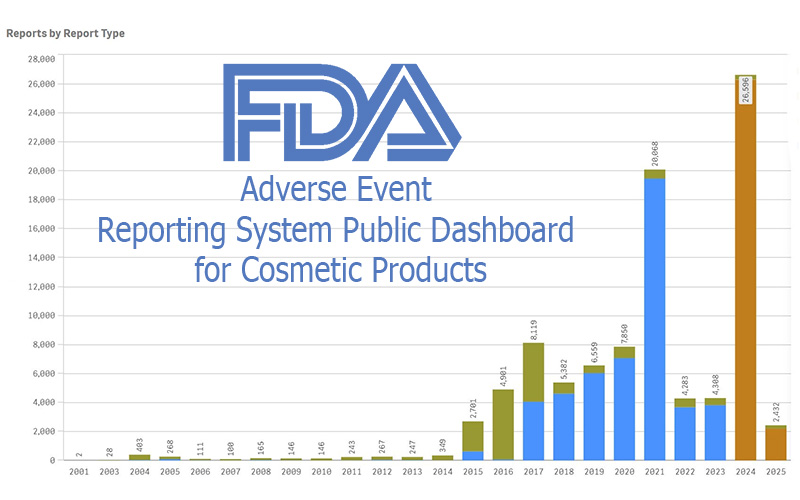
FDA Cosmetics Adverse Event Dashboard
FDA just switched on a live, public cosmetics adverse-event dashboard—big on transparency, light on verification. Here’s how it could help (or hurt) small makers.
-
On the Label: Business Name & Address
Putting your business name and address on the label isn’t just a formality—it’s a legal requirement and a mark of accountability on every product you sell.
-

Label Design: The Physical Label
Your physical label is more than packaging—it’s branding, function, and customer experience combined. Selecting the right material, finish, and adhesive ensures both beauty and durability.
-

MoCRA Rulemaking Updates
The Spring 2025 Unified Agenda, published September 4th, shows more delays in the publication of MoCRA’s fragrance allergen and GMP rules.
-

Product Identity, Product Name, and Brand
Product identity, product name, and brand each serve a different purpose on your label. Here’s how to keep them clear—and compliant.
-

Does AI Give Good Labeling Advice?
I conducted an experiment to determine if AI could help decode cosmetic regulations —but every platform I tested gave some incorrect information. Useful, yes. 100% trustworthy, no.
-

Soap: Cosmetic or Not? Understanding the Differences
Soap can be cosmetic or non-cosmetic, depending on how it’s made and marketed. Knowing the difference helps you label correctly, stay compliant, and market effectively.
-

Net Contents: Size and Placement
The net contents must be big, bold, and clear—often bigger than you’d expect. Here’s how to size, place, and format it correctly.
-

Why Are There Restrictions on Product Claims?
From snake oil salesmen to modern rules, today’s restrictions on product claims come from 100 years of experience to protect consumers—and define the line between cosmetics and drugs.
-

California Bans Plastic Glitter in Cosmetics
California has approved a ban on plastic glitter in personal care products — including soap. Makers have until 2029 to reformulate with eco-friendly alternatives.
-

Net Contents: Solid vs Fluid Products
Correct net contents labeling means using weight for solids and volume for liquids. Here’s how to measure and declare contents correctly on your cosmetic labels.
