Category: Soap & Cosmetic Labeling
Blog posts that deal with soap and cosmetic labeling; addition information, questions asked and answered and updates as new information becomes known.
-

What to Do With Soap Ends & Trimmings
Wondering what to do with those bits of soap from the end of the loaf that don’t make full sized bars? You have options!
-

Cosmetic Ingredient Look-Up
An excellent new cosmetic ingredient look-up database is online where you can look up specific ingredients, get explanations of various substance types and see typical ingredients by product type.
-

Don’t Be a Copycat
I recently went looking online for a few product pictures to use as good examples of product labels. What I found were lots of bad examples.
-

Cosmetic Warning Statements
Some cosmetic products must have specific warning statements on the label: bubble bath, yoni products, products containing alhpa or beta hydroxy acids, and some others.
-

Simple Labeling Checklist
It can seem like labeling is complicated. Use this checklist to see if you need to dig a little deeper to get it right … or if your label is okay as is.
-

They Do It…Why Can’t I?
“Wait! What! This other company does it, why can’t I??” Just because someone else is (wrongly) doing it, doesn’t mean that you can.
-
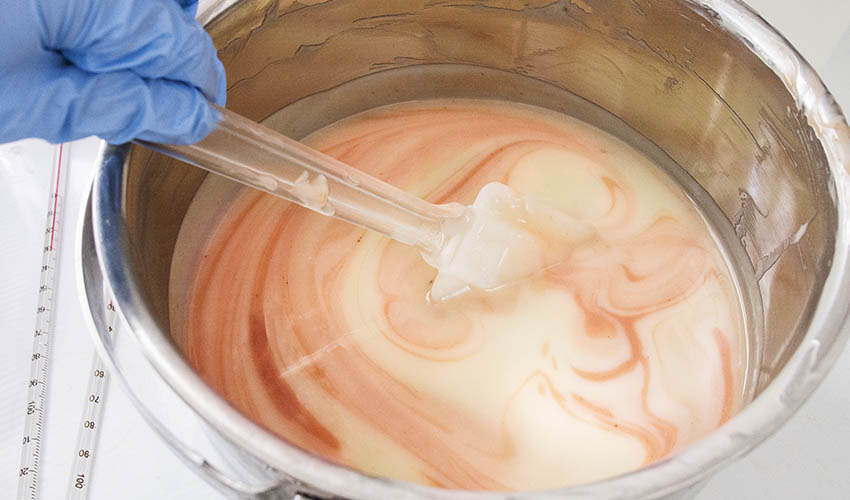
Unsaponified Oils in the Ingredient Declaration
In a soap ingredient declaration you can list what goes INTO the pot, or what comes OUT of the pot. So what do you call the unsaponified oils?
-

New Book! Navigating the Rules & Regs
New book! Navigating the Rules and Regs is finished and available at Amazon!
-

Natural Ingredients and Products
As of now (2022) the FDA has declined to provide a formal regulatory definition of the term “natural” as applied to cosmetics (or food). That said, there are some standards that can guide you concerning when the claim of “natural” is appropriate (that is, not false or deceptive) for a cosmetic product. FDA – Food […]
-

Rubbing Alcohol
The term “rubbing alcohol” originally referred to alcohols that were applied to the body rather than drinking them as a beverage. Especially during Prohibition, when consumable alcohol was illegal, the distinction was very important.1 The Alcohol and Tobacco Tax and Trade Bureau (TTB) and the United States Pharmocapeia (USP) define two different formulas for rubbing […]
-

Talc
As an ingredient in cosmetics, talc has been under scrutiny for some years now. I’ve recently done a survey of the current information about talc, and here’s what I found out. Talc was originally used as “talcum powder”—the original body or baby powder. Now it has many uses in cosmetics and other personal care products; […]
-

Made in the USA
If you want to make the claim that a product is “Made in the USA” you must comply with the Federal Trade Commission’s “Made in the USA” policy.
