This is part of the Labeling Basics series in which I am taking labeling back to its most fundamental parts, starting with the legal terms used and then going on to each requirement for soap and cosmetic labels.
Key Words to Know
Nomenclature
The words and terminology specific to a particular subject or area of study.
Botanical
Related to plants: a part of a plant.
INCI
International Nomenclature of Cosmetic Ingredients.
The International Nomenclature of Cosmetic Ingredients is a list of the standardized and internationally accepted names (called “INCI Names”) that should be used in the declaration of ingredients on cosmetic and personal care products. It is published by the Personal Care Product Council in the International Cosmetic Ingredient Dictionary and Handbook (ICID)1
The current edition of the ICID is 5 volumes, 7,000+ pages, and includes over 25,000 ingredients.
Each listing includes specific information which can be used to understand and identify that particular unique ingredient. The details include:
- Ingredient Name
- Unique INCI Monograph ID number
- CAS (Chemical Abstracts Service) Registry Number (if one exists)
- EU Chemical number
- Definition (which may include chemical formula, other scientific name(s), regulatory information, etc.)
- Chemical Class
- Reported Functions
- Ingredient Source
- Technical/Other Names
- Trade Names
- Trade Name Mixtures (where the ingredient is used in a mixture)

The “INCI Name”
When you say “This is the INCI name for ____” it means that it is the accepted and standardized name for that particular cosmetic ingredient, as it is listed in the International Cosmetic Ingredient Dictionary. There may be OTHER names for the ingredient, but the INCI name is the standardized and accepted name—the “common name” or at least the “commonly and internationally accepted name.”
Example: “Sodium bicarbonate” is the INCI name for “baking soda” or “bicarbonate of soda” or “carbonic acid, monosodium salt” (all of which are different names for exactly the same thing).
Example: “Lye” is a general term and could refer to several very different chemical substances. The correct INCI name for “lye” is either “sodium hydroxide” OR “potassium hydroxide” depending on its chemical make-up.
Botanical ingredients also have different ways of being identified. There are Latin scientific names based on the Linne classification system which are used to identify a specific plant. There are also the common names for plants, which can vary based on the country and language.
The first editions of the Cosmetic Ingredient Dictionary were for the US, and the plant-based ingredients were listed by their common English name (e.g., Fir Needle Oil). In later editions (starting in 1995), the internationally recognized Linne classification system was used to identify plants,2 in order to eliminate naming confusion across multiple languages (e.g., Abies Siberica Oil).
International
Internationally, the listings in the ICID are used as-is, including the Latin Linne-classification name for botanical ingredients.
United States
In the United States, the Fair Packaging and Labeling Act requires ingredients be listed by the “common or usual name of each ingredient.”3 The regulations specifically reference the 2nd edition (1977)4, but later publications by the FDA indicate that more recent editions of the ICID should be referenced to identify the correct name to be used for cosmetic ingredients.5
When the ICID was first updated to use the Latin names for botanical ingredients (6th edition, 1995), the publishers petitioned the FDA to allow the new “harmonized” (international) names for botanical ingredients.
The FDA responded:
“We cannot accept the current… proposal that the genus/species names, using the Linne system in the primary position with the English plant name in parenthesis, be allowed for the purpose of declaring botanical ingredients on the labels of products intended for distribution and sale in the United States.“
FDA Response
Therefore, in the United States, even though other types of ingredients should use the INCI name, botanical ingredients should be listed by the common English name.
The FDA also stated that they would be “unlikely to object” if a petition was submitted to list botanicals by their common English name with the Linne INCI name in parenthesis.6
INCI Name Rules for Ingredient Declaration
- Use the INCI name from the International Cosmetic Ingredient Dictionary. (Examles: Sodium Bicarbonate or Water.)
- EXCEPT in the United States, use the common English name for plant-based (botanical) ingredients. (Examples: Lavender Oil or Coconut Oil.)
The INCI name for plant-based ingredients may be stated in parentheses:
Lavender (Lavendula Angustfolia) Oil or Coconut (Cocos Nucifera) Oil
Reality vs. Regulation
The 16th edition of the Cosmetic Ingredient Dictionary came out in 2016. There hasn’t been a print edition since then, although it has been updated online. Since that time the FDA hasn’t updated their regulations on the subject, but the United States and global cosmetic industry have gradually adopted the international Latin Linne naming system for botanical ingredients. Most of the products you see on retail shelves use the INCI (Latin) name first and sometimes (but not always) include the common English name.
In my many years of monitoring the warning letters from the FDA, I have never seen them cite a failure to use the common English name first. Nor does it appear that imported cosmetics are required to list the common English name first for botanical ingredients.
In fact, even back in 1995 in the letters the FDA sent responding to requests to update the regulations, they cited several food-related cases where parenthetical names were initially allowed and then after 5 years or so those parenthetical names became the allowed names. According to the letters, it was based on “consumer familiarity” with the new names.
So, while nothing has been officially stated and the regulations haven’t changed, it looks like the FDA is, in fact, accepting the INCI naming for botanical ingredients. Consumers are now familiar with Latin names, and even expect them. Given that the FDA has been participating in International Cooperation on Cosmetics Regulation, it wouldn’t be surprising to see some sort of official clarification by the FDA on the use of INCI names for botanical ingredients at some point in the future.
Cosmetic Ingredient Dictionary – General Naming Rules
Trade Name or Brand Name
The INCI name of an ingredient is not the “trade name” or the “brand name” of the ingredient. Just because you refer to your lye as “Red Devil” (and maybe it IS a red devil), that’s not the correct name for the ingredient declaration. In that case the common name would be the correct chemical name, “sodium hydroxide.”
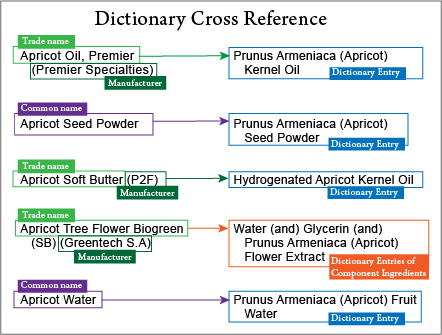
In some cases, a trade name is registered to be included in the ICID (for a fee) and can be looked up in the ICID cross-reference to find what the INCI name is for that ingredient. (See image below for examples.)
Blended Ingredient
Some ingredients are actually blends of several components. Preservatives are a good example of this. If the product is a blended ingredient, the supplier should be able to tell you the common (“INCI”) names for the individual components.
When the product, such as Germall Plus, is registered and listed in the ICID cross-reference it will show as (for Germall Plus), “Propylene Glycol (and) Diazolidinyl Urea (and) Iodopropynyl Butylcarbamate.”
Sometimes suppliers will say, for example, “The INCI name is: Propylene Glycol (and) Diazolidinyl Urea (and) Iodopropynyl Butylcarbamate.” However, that’s isn’t actually the INCI Name for the product! It is the listing of the component ingredients by their INCI Names. Each one must be listed individually in the ingredient declaration. Using “(and)” is not allowed in cosmetic ingredient declarations.
Names Established by Other Entities
Where ingredient names and specifications were established previously by other entities, those are normally included in the ICID. Other entities include the U.S. Pharmacopaeia (USP), National Formulary (NF), Food Chemicals Codes (FCC), United States Adopted Names (USAN) and others.
Emulsifying Wax
One interesting exception is Emulsifying Wax NF, which is defined in the National Formulary but is not listed in the ICID. It appears that you can use the term “Emulsifying Wax NF” in your ingredient declaration if the product you are using meets the National Formulary specifications.
Infusions, Teas, & Tinctures
When an ingredient is an infusion, tea, tincture, or other combination of a botanical ingredient and a liquid (solvent), the material thus extracted from the plant is called an “extract.” It is listed in the ingredient declaration along with the liquid in the same manner as a blended ingredient; the liquid and the extract are listed separately, in descending order of predominance in the whole.
As a general rule, the amount of actually extracted botanical material in an infusion, tea, or tincture (after straining) is relatively small and very difficult to measure. The good news is that it is likely to be less than 1% of the whole, so may be listed with other ingredients present at 1% or less in any order, following the ingredients present at more than 1%.
Example: Calendula infused olive oil. After the calendula is strained out, you are left with olive oil and calendula extract. The olive oil is listed based on the percentage in the whole and the calendula extract could be included in any order with the ingredients present at 1% or less.
Water
The INCI name for fresh water is simply “water” regardless of the source (spring water, river water, glacier water) or purity (distilled, purified, sterile). It is just listed as “water.”
Sea water is listed as “sea water.”
Water that is the result of the distillation process of plants is listed by the name of the plant and “water” (e.g., Lavender water).
Salt
Salt, generally, is listed as “sodium chloride” and is defined as a synthetic product.
“Sea salt” is the correct name for salt that is derived from sea water or inland bodies of salt water. Salt from the Dead Sea would be identified as “sea salt.” Pink salt is also another name for sea salt (so it would also be listed as “sea salt” in the ingredient declaration).
Where Do You Find The INCI Name?
The complete ICID with all the details is not available for free on-line. There is a subscription service, $525 per year for members of the Personal Care Product Council; $1,250 annual subscription fee for non-members. The published 5-volume set is $1,250 for non-members.
If you don’t need all 25,000 ingredients and the other information contained in the books or online subscription, your should be able to get the correct INCI name for your ingredients from your supplier.
If you are using ingredients you purchase retail or can’t otherwise locate the correct name, there are several INCI look-ups online which contain the most common ingredients. One good place to to start is the Handcrafted Soap & Cosmetic Guild’s Ingredient Lookup. Note that you have to register in order to access the HSCG Ingredient Lookup, but it is free.
Finally, you can check the Wikipedia or do a Google search for the ingredient name.
Why is it Important?
Correctly identifying the ingredients in your products is required by law and by regulations.
It is also a service to your customers to give them enough data to make an informed decision about the value of your product and whether they want to purchase it.
Using the established names for ingredients provides transparency and truthfulness, making the data understandable. It also makes it possible to compare different products because the same names are used to identify the ingredients.
All in all, using the correct names to identify the ingredients in your products presents you as a professional in your field.
- First published as the Cosmetic Ingredient Dictionary (1973) it was for the US only. The name was changed to the International Cosmetic Ingredient Dictionary in 1995 when it was accepted by the European Council as the standard for EU cosmetic ingredient declarations. ↩︎
- The INCI name is USUALLY the same as the name in the Linne classification system, but not always. Where names or classifications have changed over time (and they do!) the INCI name may not be the same as the name currently accepted in the botanical scientific community. ↩︎
- 15 USC 1454 (c)(3)(B) ↩︎
- 21 CFR 703(c)(2)(i) ↩︎
- Current Issues in the Safety and Labeling of Cosmetics; Linda Katz, Office of Cosmetics and Colors (video), Oct 20, 2011. https://www.youtube.com/watch?v=akPq1tsbze0 . ↩︎
- There’s no indication that such a petition was ever submitted, but it has since become commonplace to put the Linne INCI name in the ingredient declaration. ↩︎




Leave a Reply